A gene mutated in tumors allows cells to " gear up " and spread
This text is a translation of the text published in French on the CNRS website.
Cells can migrate throughout the human body. In the embryo, migrations are critical for organ development. In the adult, only a few cells retain this migration ability, for example the immune cells that patrol the body to fight infections. However, during cancer progression, initially non-migrating tumor cells find a way to disseminate and form metastases far away from the primary tumor. At this stage, cancers become particularly dangerous. They can no longer be treated by surgery and require chemotherapy.
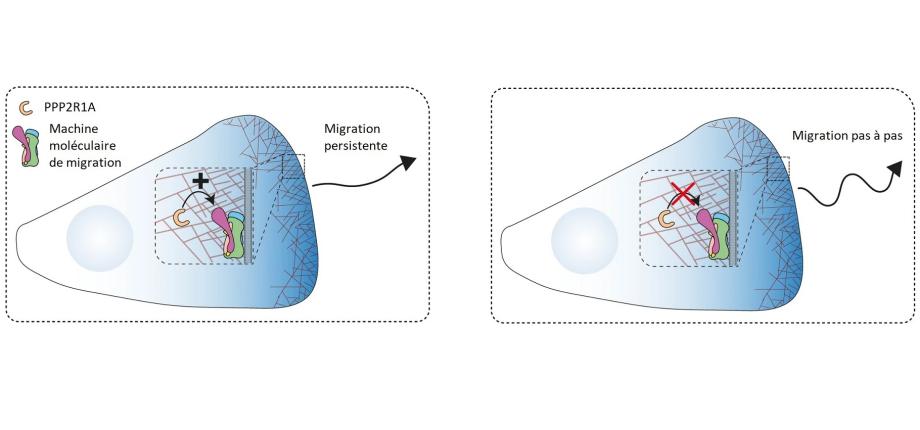
Scientists have turned their attention to the machinery of cell migration, that is all the molecular machines that fit together like cogs and allow cells to move forward. We know already how these molecular machines enable the cell to take one step, but not how they are coordinated to allow the cell to "walk". Yet it is the persistence of the process, i.e. the repetition of steps, that allows cells to migrate efficiently and tumour cells to spread. In this study, scientists looked for the proteins that bind to an essential migration machine, when cells walk or just take a step. This approach identified PPP2R1A, the product of a gene mutated in various gynecological cancers: breast, endometrium and ovaries. This gene is required for persistent migration, but not for taking a single step.
The scientists thus understood that PPP2R1A changes one of the molecular machines involved, as when a car driver gears up. When the persistent mode is engaged, the active protein of the machine frees itself from an inhibitory constraint to allow successive steps. Meanwhile, its place is taken by a similar protein. This similar protein is likely to allow the free protein to regain its place when the walk is over. Scientists have shown that PPP2R1A only regulates migration persistence if this alternative machinery mechanism is engaged. Without this mechanism, the walk stops soon, since the cell doesn't take another step. However, scientists have succeeded in forcing the cell to walk without this mechanism, but in this case, a new impulse must be given for each step. Importantly, the mutations found in patients' tumors abrogate the ability of PPP2R1A to perform persistent migration. Further insights into the mechanisms that regulate migration persistence, however, are required before scientists can identify effective ways of preventing the spread of tumor cells.













